Importance of Hydroxylamine And Its Derivatives - by Sri Hari Sundararajan

b'The compound hydroxylamine is remarkably close in structure to ammonia, and differs only in containing an additional hydroxyl, thereby giving it basic properties. Normally a crystalline compound at room temperature, this base is also known to play significant roles as a reducing agent as well as in imine formation.
Imines are highly reactive nitrogen double-bond containing compounds that are formed through an addition reaction with a carbonyl group in acidic conditions (aldehyde/ketone): 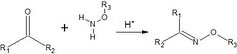
Despite their rather explosive nature, hydroxylamines are useful compounds in themselves or in their other derived forms.
For more information on hydroxylamines, click on this link.
1). Hydroxyammonium Nitrate
Being the salt of hydroxylamine and nitric acid (via an acid-base reaction), this corrosive and toxic substance is held in high regard by rocket scientists, as this compound is considered to be a potential rocket propellant. The following shows the synthetic mechanism of creating this compound: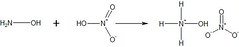
Click on this link for more information on the properties of the energetic compound hydroxyammonium nitrate and its significant use as a rocket propellant.
Click on this link directing to a Chem 243 lecture describing the various reactions of aldehydes and ketones (including imine formation).
2). Separation of Aldehydes and Ketones using Oxime Derivatives
In the case of hydroxylamines, the resulting imine products that are formed from their interaction with a carbonyl in acidic conditions results in the formation of a special subset of imines known as oximes (from aldehyde \\u2013 aldoxime/from ketone \\u2013 ketoxime). Oximes are normally colorless crystalline compounds. In fact, it is this property of these compounds that allow for their usage in determining the identity of the respective aldehyde/ketone that the hydroxylamine originally reacted with. In the following example, the use of the hydroxylamine derivative para-nitrobenzyl hydroxylamine is reacted with the respective carbonyl compound. As a result, the new compound is easily able to be detected and identified through separation techniques such as High Performance liquid Chromatography (HPLC), as the nitro groups increase molar absorptivity and increased sensitivity. In addition, alkylated oxime groups are more easily seperated by the technique HPLC than standard ones.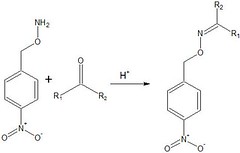
As the peer article referred to didn\\u2019t provide a mechanism as to how the p-nitrobenzyl hydroxylamine was synthesized, I have provided a sample mechanism that leads to its production: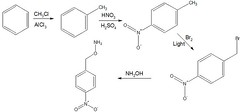
This link leads to the respective peer review article referred to in obtaining this information.
Click on one of the following three links that direct to Chem 243 lectures for information on:
A) Electrophillic Aromatic Substituion (use of Friedel-Crafts Alkylation in order to add a methyl group to a benzene ring).
B) Ortho/Para Vs. Meta Directing Groups (ability of methyl group on benzene ring to direct the respective nitro group being added on through a nitration reaction to a para position).
C) Side Chain Reactions of Aromatic Compounds and introduction to Aldehyde and Ketone properties (substitution of methyl\'s hydrogen on the benzyl position with bromine through free radical halogenation).
3. Synthesis of Caprolactam
Caprolactam is one the many monomeric subunits used in the overall synthesis of the material Nylon-6. Once an imine synthetic reaction is performed on a cyclohexanone with a hydroxylamine, the resulting product can be converted into caprolactam through a specific reaction known as the Beckmann Rearrangement. The Beckmann Rearrangement is an acid catalyzed reaction that allows for the conversion of an imine into an amide, as seen below: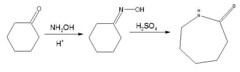
For more information on the synthetic properties of the formed caprolactam, please click on this link.
Compound Info
Hydroxylamine: PubChem 789 hydroxylamine Oxammonium Oxyammonia Nitroxide NH2OH
ChemACXnet to purchase ACX number X1008257-2 CAS number 7803-49-8'